Enlivex Therapeutics Ltd. (Nasdaq: ENLV) is a clinical-stage macrophage reprogramming immunotherapy company developing next-generation treatment options to combat osteoarthritis and inflammatory diseases. The company is advancing several clinical programs, evaluating Allocetra™, a universal, off-the-shelf cell therapy platform designed to restore immune balance, in treating knee osteoarthritis, basal thumb joint (first carpometacarpal joint) osteoarthritis sepsis as well as other inflammatory diseases. To learn more about this innovative company and how it plans to impact treatment markets, Hawk Point Media reached out to Enlivex Therapeutics CEO Oren Hershkovitz, Ph.D., to get a firsthand account of expectations for 2024. Here's what Mr Hershkovitz said:
Q. Enlivex Therapeutics is in a biotech segment described as "next-gen" science. What does that mean, and how did you earn that descriptive?
A. It means we are doing things that are at the forefront of science that others don’t do. And we got here through innovation and perseverance. More specifically, Enlivex Therapeutics has evolved into a clinical-stage company that is advancing one of the most innovative and potentially effective immunotherapy technologies known: macrophage reprogramming.
Q. What is a macrophage?
A. Macrophages are a type of white blood cell that plays a critical role in the human immune system, particularly in facilitating vital functions such as engulfing and digesting microorganisms. These macrophages are crucial to overall health because they clear out debris and dead cells and stimulate other cells to get involved in immune function.
Q. These need reprogramming?
A. Yes, in many cases. While the human body is a marvel, it sometimes needs help to treat diseases and other medical conditions. Actually, in many diseases, macrophages drive an immune inflammatory acute or chronic state that is harmful. Therefore, reprogramming critical immune defenses and returning them to do their jobs more effectively is essential to treat the disease We are providing that ability by advancing what we believe will be a breakthrough treatment to the sector with Allocetra™, an immunotherapy aimed at reprogramming macrophages to treat various diseases. We are on the path to proving that after publishing positive interim results from a Phase I/II trial for knee osteoarthritis and dosing the first patient in a Phase I/II trial for thumb osteoarthritis..
Q. Why did you target these conditions?
A. For several reasons. Firstly, immunotherapy treatment for patients with those diseases is critically needed, noting that both conditions can be highly debilitating over time and are currently underserved and, in many cases, unmet medical needs. Second, before entering the clinic, our work showed that Allocetra™ could be an excellent treatment option based on its demonstrated ability to reprogram macrophages to treat inflammatory and autoimmune diseases. And thirdly, we can provide Allocetra™ as a universal, cost-effective off-the-shelf cell therapy with a simple manufacturing process. That third point is crucial because it will allow us to scale quickly, get effective treatment to patients, and begin generating ROI in as little as days after approval, not months. These are all multi-billion dollar markets with substantial unmet medical needs.
Keep in mind that we have already published positive interim data from a Phase I/II end-stage knee osteoarthritis (OA) trial and have announced that we have received regulatory authorization for the Phase I/II thumb osteoarthritis trial. So, we are working every day to reach those endpoints.
Q. What else is in the pipeline?
A. Quite a bit. We are advancing our Phase 2 clinical program, evaluating our treatments to combat organ failure associated with sepsis. And we are enrolling patients for our Phase 1/2 moderate knee, end-stage knee, and basal thumb osteoarthritis trials. We are also working vigorously to advance additional projects into clinical studies in other local chronic inflammatory diseases.
We have already published positive results in treating high-risk urosepsis, severe and critical COVID-19, and vanishing bone disease. Those publications highlight the role of apoptotic cells in immune homeostasis. That is important, especially related to sepsis and osteoarthritis, because there are currently no FDA/EMA-approved drugs to cure these diseases; current treatments only address complications, not the core immune response.
Allocetra™ is showing potential to address this unmet medical need, positioning it well to target a $33 billion global market for severe sepsis. Furthermore, because it is a cost-effective, novel therapeutic that can modulate and reprogram macrophages, we believe it can earn first-line designation to treat targeted conditions.
Q. You mentioned trials are underway. Can you provide more insight?
A. Yes, we have several ongoing trials. And the data is compelling. We just published interim results from end-stage osteoarthritis patients reporting substantial pain relief 3 months following AllocetraTM treatment. Results from our Phase I/II sepsis trial showed a potential indication of effect in the sepsis population of high-risk urinary tract infections. We have an ongoing placebo-controlled, randomized, double-blind trial in up to 160 patients with knee osteoarthritis and a phase I/II placebo-controlled, randomized, double-blind trial in up to 46 patients with basal thumb osteoarthritis. In our Phase I/II sepsis study, we saw a significant improvement in patient outcomes. Filling its role, Allocetra™ led to complete organ recovery in all treated patients by day 28. The results demonstrated statistically significant improvement in hospitalization duration and Sequential Organ Failure Assessment (SOFA) scores, which was coupled with a favorable safety profile in treated patients.
Q. You actually highlighted milestones in a recent CEO letter. Can you tell us more about that?
A. That letter highlighted achievements, including completing numerous pre-clinical studies, infusing Allocetra™ in over 100 patients and showing a favorable safety profile and potential efficacy, and provided an overview of results from our Phase 2 sepsis trial showing a potential effect and favorable safety profile, particularly in high-risk urinary tract infection sepsis patients.
Also highlighted was having cash resources on hand after completing our $15 million financing round to cover expected key trial milestones, including those related to our ongoing knee osteoarthritis and basal thumb trials. Milestones from those are anticipated in Q4 2024 as well as each quarter during 2025.
Q. You did announce interim results from the Knee Osteoarthritis trial, correct?
A. We did, and they were excellent, which is not going unnoticed. Remember, knee osteoarthritis is highly prevalent and disabling, with no approved treatments to slow or reverse the disease's progression. Our trial design investigated Allocetra™ as a "last resort" alternative to knee replacement surgery. Interim results showed an average pain reduction in patients of 64%; 33% reported complete pain relief, and 89% avoided knee replacement surgery. Furthermore, no severe adverse events were reported, adding to the case for Allocetra™ to become a first-line, not a "last resort" treatment.
Q. It appears Enlivex is making operational and clinical strides across the board. How do you think 2024 and 2025 will play out?
A. We certainly have a filled agenda as we work toward delivering better treatment solutions for osteoarthritis and other inflammatory diseases. We have several ongoing trials, encouraging results from our lead product, Allocetra™, which could become a game-changer in treating degenerative joint diseases, and the capital to accelerate programs toward meeting endpoints, actually all the way to the end of 2026.
Let me end with this: Enlivex is not following the clinical herd. It is blazing a new trail for better treatment options with a drug that may provide relief for millions suffering from debilitating osteoarthritis conditions. If trial precedent holds, Enlivex could be a vital contributor to improving pain management and joint function and reducing the need for joint replacements. We are on the right track to deliver those results. In other words, 2024 can be a transformational year for this company.
End interview.
Pipeline:
Source: Enlivex Therapeutics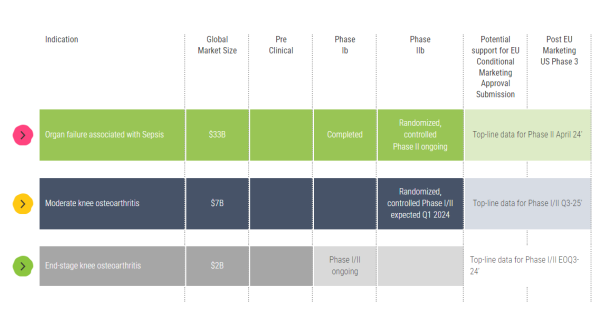
Read the June 2024 Enlivex Therapeutics company presentation by clicking HERE.
ABOUT ALLOCETRA™
Allocetra™ is being developed as a universal, off-the-shelf cell therapy designed to reprogram macrophages into their homeostatic state. Diseases such as solid cancers, sepsis, and many others reprogram macrophages out of their homeostatic state. These non-homeostatic macrophages contribute significantly to the severity of the respective diseases. By restoring macrophage homeostasis, Allocetra™ has the potential to provide a novel immunotherapeutic mechanism of action for life-threatening and life-debilitating clinical indications that are defined as “unmet medical needs.”
ABOUT BASAL THUMB OSTEOARTHRITIS
Osteoarthritis of the thumb is a chronic condition causing pain, stiffness and occasional clicking and swelling in the joint at the base of thumb (also known as the carpometacarpal or CMC joint). Simple daily tasks can become painful and difficult. The prevalence of thumb osteoarthritis increases substantially with age and is more common in postmenopausal woman. The prevalence of radiographic base thumb OA was reported to be 5.8% and 7.3%, for 50-year-old males and females, respectively, while the respective prevalence for 80-year-old male and female participants was reported as 33.1% and 39.0%1. The overall estimated symptomatic prevalence is up to 15% in adults over 30 years of age2. Osteoarthritis of the thumb is a degenerative and progressive condition, and over time, conservative treatments and anti-inflammatory medication to reduce pain and swelling start losing their effectiveness. Currently, there are no effective long-term treatments for this disease.
ABOUT ENLIVEX
Enlivex is a clinical stage macrophage reprogramming immunotherapy company developing Allocetra™, a universal, off-the-shelf cell therapy designed to reprogram macrophages into their homeostatic state. Resetting non-homeostatic macrophages into their homeostatic state is critical for immune system rebalancing and resolution of life-threatening conditions. For more information, visit http://www.enlivex.com.
Safe Harbor Statement: This press release contains forward-looking statements, which may be identified by words such as “expects,” “plans,” “projects,” “will,” “may,” “anticipates,” “believes,” “should,” “would,” “could,” “intends,” “estimates,” “suggests,” “has the potential to” and other words of similar meaning, including statements regarding expected cash balances, market opportunities for the results of current clinical studies and preclinical experiments, the effectiveness of, and market opportunities for, ALLOCETRATM programs. All such forward-looking statements are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Investors are cautioned that forward-looking statements involve risks and uncertainties that may affect Enlivex’s business and prospects, including the risks that Enlivex may not succeed in generating any revenues or developing any commercial products; that the products in development may fail, may not achieve the expected results or effectiveness and/or may not generate data that would support the approval or marketing of these products for the indications being studied or for other indications; that ongoing studies may not continue to show substantial or any activity; and other risks and uncertainties that may cause results to differ materially from those set forth in the forward-looking statements. The results of clinical trials in humans may produce results that differ significantly from the results of clinical and other trials in animals. The results of early-stage trials may differ significantly from the results of more developed, later-stage trials. The development of any products using the ALLOCETRATM product line could also be affected by a number of other factors, including unexpected safety, efficacy or manufacturing issues, additional time requirements for data analyses and decision making, the impact of pharmaceutical industry regulation, the impact of competitive products and pricing and the impact of patents and other proprietary rights held by competitors and other third parties. In addition to the risk factors described above, investors should consider the economic, competitive, governmental, technological and other factors discussed in Enlivex’s filings with the Securities and Exchange Commission, including in the Company’s most recent Annual Report on Form 20-F filed with the Securities and Exchange Commission. The forward-looking statements contained in this press release speak only as of the date the statements were made, and we do not undertake any obligation to update forward-looking statements, except as required under applicable law.
Disclaimers: This presentation has been created by Hawk Point Media Group, Llc. (HPM) and is responsible for the production and distribution of this content. Hawk Point Media Group, LLC. has not been compensated to create this content. This content may be used and syndicated beyond the channels used by Hawk Point Media, Llc. This disclaimer must be part of all reproductions and re-publications . HPM holds ZERO shares and has never owned stock in Enlivex Therapeutics stock. The information in this video, article, and related newsletters is not intended to be, nor does it constitute, investment advice or recommendations. Hawk Point Media Group, Llc. strongly urges you to conduct a complete and independent investigation of the respective companies and consider all pertinent risks. Readers are advised to review SEC periodic reports: Forms 10-Q, 10K, Form 8-K, insider reports, Forms 3, 4, 5 Schedule 13D. Never take opinions, articles presented, or content provided as the sole reason to invest in any featured company. Investors must always perform their own due diligence before investing in any publicly traded company and understand the risks involved, including losing their entire investment.
Media Contact
Company Name: Hawk Point Media
Contact Person: Editorial Dept.
Email: info@hawkpointmedia.com
Country: United States
Website: https://hawkpointmedia.com/














