- Significant improvement in balance after four-week treatment with AM-125 20 mg nasal spray vs. placebo
- Study provides proof-of-concept for enhancement and acceleration of vestibular recovery
- Treatment well-tolerated by study participants and safe
- Company plans a US FDA IND filing, and to partner AM-125
- Altamira sees opportunity to provide novel treatment option with vestibular stimulant in therapeutic area neglected for decades
HAMILTON, BERMUDA / ACCESSWIRE / June 13, 2022 / Altamira Therapeutics Ltd. (NASDAQ:CYTO), a company dedicated to developing therapeutics that address important unmet medical needs, today announced positive top-line data from its exploratory Phase 2 TRAVERS trial with AM-125 (intranasal betahistine) in acute vertigo and provided an update on its development plans.
The randomized, double-blind, placebo-controlled TRAVERS trial enrolled at more than ten study sites across Europe a total of 124 patients who suffered from acute vertigo following neurosurgery for the removal of a tumor. Patients were randomized to receive either AM-125 at up to 20 mg or a placebo three times daily for four weeks, which was followed by a two-week treatment-free observation period. In addition, all trial participants followed a standardized course of vestibular rehabilitation therapy. Improvement in the "Tandem Romberg" test, which measures how long patients are able to maintain balance with their two feet aligned one after the other while they have their eyes closed, served as the primary efficacy outcome. For reference, the trial also included 16 patients who received ‘open label' oral betahistine at 16 mg three times daily (the approved dose in most countries worldwide).
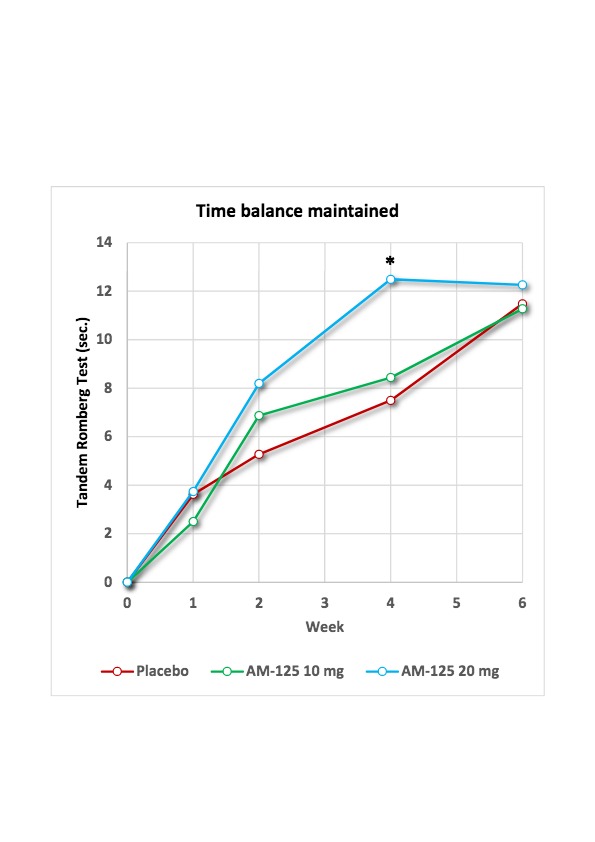
The TRAVERS trial demonstrated good safety and tolerability of AM-125 at doses up to 20 mg administered three times daily for four weeks. Further, administration of AM-125 resulted in a dose- and time-dependent improvement in balance and vestibular function. At the end of the treatment period, patients treated with AM-125 20 mg on average managed to maintain balance for 12.5 seconds vs. 7.5 seconds for placebo treated patients, which is a statistically significant improvement (p=0.0242; least square means in repeated-measure ANCOVA model, per protocol population). While the active group maintained this level during the subsequent observation period, the placebo group continued its spontaneous recovery course and ultimately converged with the former, resulting in a miss of the primary endpoint which had been set at 6 weeks.
See Chart 1 below: Improvement in seconds of balance maintained during the Tandem Romberg test from baseline (least square means in repeated measures ANCOVA model) in the TRAVERS trial. * denotes a statistically significant difference <0.05.
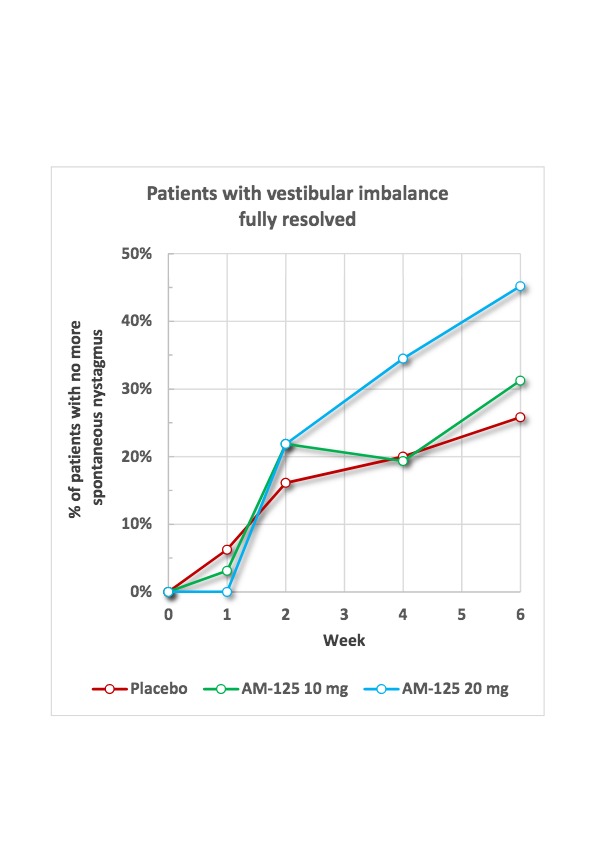
Spontaneous eye movements (nystagmus), a hallmark and objective indicator of vestibular imbalance and vertigo, had fully resolved in 34.5% of the AM-125 20 mg group after the treatment period and in 45.2% after six weeks compared with 20.0 and 25.8%, respectively, in the placebo group.
See Chart 2 Below: Percentage of trial participants with full resolution of vestibular imbalance as determined by complete absence of spontaneous eye movements (nystagmus) in the TRAVERS trial.
"When the peripheral vestibular system suddenly loses its function, patients will experience acute vertigo and difficulties in maintaining balance even for walking a few steps," commented Paul van de Heyning, MD, Professor, Department of Translational Neurosciences, Faculty of Medicine and Health Sciences, University of Antwerp, Belgium, who served as the International Coordinating Physician of the TRAVERS trial. "The brain reacts with central vestibular compensation to progressively mitigate this imbalance and physical rehabilitation exercises help to improve this process. So far, no drug treatment has been shown to be truly effective in accelerating and enhancing this vestibular compensation, which is why the results obtained with AM-125 in the TRAVERS trial appear to be very promising."
"We are strongly encouraged by the key outcomes from the TRAVERS trial, which confirm the good safety and tolerability of AM-125, demonstrate its therapeutic activity and thus provide the first proof of concept in a model representative for various types of acute vestibular syndrome," commented Thomas Meyer, Altamira Therapeutics' founder, Chairman and CEO. "For patients suffering from acute vertigo, regaining balance as quickly as possible is of utmost importance. AM-125 stimulates vestibular compensation, thereby helping patients to ‘get back on their feet' more quickly, which is what we have demonstrated in the TRAVERS trial. I would like to extend my sincere thanks to all the patients and investigators involved in the TRAVERS trial who helped us to reach this important milestone. We look forward to advancing the AM-125 program and to bringing this innovative nasal spray to patients suffering from acute vertigo."
Following further analyses, the study team will publish detailed results in a scientific journal. Meanwhile, the Company is planning to move the AM-125 development program forward by filing an Investigational New Drug (IND) application with the US Food and Drug Administration (FDA). The data and insights from the TRAVERS trial - e.g. into the time course of vestibular recovery - together with regulatory feedback will help to inform the design and conduct of the next clinical trial. The Company expects to initiate that next trial later this year. As it has previously reported, Altamira intends to partner the AM-125 program.
Globally, there has been a dearth of innovation for treating vertigo for decades. More than four out of ten Americans, at some point in their lives, experience an episode of dizziness significant enough to see a doctor. Current drug-based treatment options include vestibular suppressants such as benzodiazepines or centrally acting antihistamines, which only treat short-term symptoms such as nausea, and (oral) betahistine as a vestibular stimulant. Like the other drugs, oral betahistine was introduced decades ago. It is used as a standard of care in many countries around the world, representing about $450 million in revenues, but not approved in the US and is limited in its clinical utility due to poor oral bioavailability. Although not directly comparable due to the lack of placebo control, in TRAVERS the oral betahistine group showed a clear and consistent trend for inferior activity across efficacy measures when compared with the intranasal betahistine group, in particular AM-125 20 mg. The TRAVERS clinical trial is an important step in the clinical development of AM-125 with the goal of providing a safe and effective novel treatment option for the treatment of acute vertigo triggered by neurosurgery or various other injuries to the peripheral vestibular system.
About Betahistine
Betahistine, a small molecule structural analog of histamine, acts as an agonist at the H1 histamine receptor and as an antagonist at the H3 histamine receptor. Unlike histamine, it crosses the blood-brain-barrier. Betahistine is known to increase the release of histamine, acetylcholine, dopamine and norepinephrine in the brain. It increases cochlear, vestibular and cerebral blood flow and facilitates vestibular compensation and inhibits neuronal firing in the vestibular nuclei. Betahistine for oral administration is approved in about 115 countries (with the U.S. being a notable exception) for the treatment of vertigo and Meniere's disease. Despite its good safety profile, the clinical utility of orally administered Betahistine is limited due to poor bioavailability.
About AM-125
AM-125 is an intranasal formulation of betahistine. Because of its ability to circumvent first-pass-metabolism, AM-125 has been shown to have 5-to-29 times higher bioavailability than orally administered betahistine. Altamira Therapeutics is developing AM-125 for the treatment of acute vertigo. With its incidence and prevalence increasing with age, vestibular dysfunction affects more than one third of the U.S. population 40 years of age and older.
About Altamira Therapeutics
Altamira Therapeutics (NASDAQ:CYTO) is dedicated to developing therapeutics that address important unmet medical needs. The Company is currently active in three areas: the development of RNA therapeutics for extrahepatic therapeutic targets (OligoPhore™ / SemaPhore™ platforms; preclinical), nasal sprays for protection against airborne viruses and allergens (Bentrio™; commercial) or for the treatment of vertigo (AM-125; Phase 2), and the development of therapeutics for intratympanic treatment of tinnitus or hearing loss (Keyzilen® and Sonsuvi®; Phase 3). Founded in 2003, it is headquartered in Hamilton, Bermuda, with its main operations in Basel, Switzerland. For more information, visit: https://altamiratherapeutics.com/
Forward-Looking Statements
This press release may contain statements that constitute "forward-looking statements" within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Forward-looking statements are statements other than historical facts and may include statements that address future operating, financial or business performance or Altamira Therapeutics' strategies or expectations. In some cases, you can identify these statements by forward-looking words such as "may", "might", "will", "should", "expects", "plans", "anticipates", "believes", "estimates", "predicts", "projects", "potential", "outlook" or "continue", or the negative of these terms or other comparable terminology. Forward-looking statements are based on management's current expectations and beliefs and involve significant risks and uncertainties that could cause actual results, developments and business decisions to differ materially from those contemplated by these statements. These risks and uncertainties include, but are not limited to, the approval and timing of commercialization of AM-301, Altamira Therapeutics' need for and ability to raise substantial additional funding to continue the development of its product candidates, the timing and conduct of clinical trials of Altamira Therapeutics' product candidates, the clinical utility of Altamira Therapeutics' product candidates, the timing or likelihood of regulatory filings and approvals, Altamira Therapeutics' intellectual property position and Altamira Therapeutics' financial position, including the impact of any future acquisitions, dispositions, partnerships, license transactions or changes to Altamira Therapeutics' capital structure, including future securities offerings. These risks and uncertainties also include, but are not limited to, those described under the caption "Risk Factors" in Altamira Therapeutics' Annual Report on Form 20-F for the year ended December 31, 2021, and in Altamira Therapeutics' other filings with the SEC, which are available free of charge on the Securities Exchange Commission's website at: www.sec.gov. Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those indicated. All forward-looking statements and all subsequent written and oral forward-looking statements attributable to Altamira Therapeutics or to persons acting on behalf of Altamira Therapeutics are expressly qualified in their entirety by reference to these risks and uncertainties. You should not place undue reliance on forward-looking statements. Forward-looking statements speak only as of the date they are made, and Altamira Therapeutics does not undertake any obligation to update them in light of new information, future developments or otherwise, except as may be required under applicable law.
CONTACT
Investors@altamiratherapeutics.com
800-460-0183
SOURCE: Altamira Therapeutics Ltd.
View source version on accesswire.com:
https://www.accesswire.com/704821/Altamira-Therapeutics-Announces-Top-Line-Data-from-AM-125-Phase-2-Study-in-Acute-Vertigo













