Study will investigate potential activity of a novel immunotherapeutic (LYT-200) with checkpoint inhibition as a new treatment modality
Topline Phase 1 results with single-agent LYT-200 expected in the fourth quarter of 2021
PureTech Health plc (Nasdaq: PRTC, LSE: PRTC) (“PureTech” or the “Company”), a clinical-stage biotherapeutics company dedicated to discovering, developing and commercializing highly differentiated medicines for devastating diseases, today announced a clinical trial and supply agreement with an affiliate of BeiGene, Ltd. (Nasdaq: BGNE; HKEX: 06160) to evaluate BeiGene’s tislelizumab, an anti-PD-1 immune checkpoint inhibitor, in combination with PureTech’s LYT-200, an investigational monoclonal antibody targeting galectin-9, for the potential treatment of difficult-to-treat solid tumor indications that are associated with poor survival rates.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20210707005110/en/
PureTech announced a clinical trial and supply agreement with BeiGene to evaluate BeiGene’s tislelizumab, an anti-PD-1 immune checkpoint inhibitor, in combination with PureTech’s LYT-200, an investigational monoclonal antibody targeting galectin-9, for the potential treatment of difficult-to-treat solid tumor indications that are associated with poor survival rates. (Photo: Business Wire)
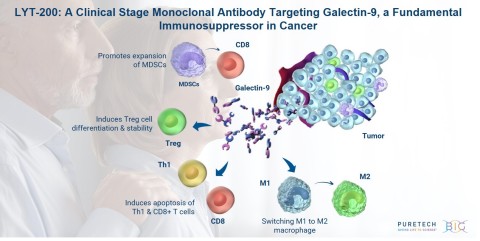
Galectin-9 is a pivotal immune modulator widely expressed in multiple difficult-to-treat tumor types and involved in the regulation of tumor promoting inflammatory and immunosuppressive pathways. Inhibition of galectin-9 with targeted antibodies leads to upregulation of immunostimulatory cytokines and anti-tumor activity in preclinical cancer models. LYT-200 is currently being evaluated as a single agent in the first phase of an adaptive Phase 1/2 clinical trial, which, based upon the trial protocol, will be followed by the portion of the trial intended to investigate LYT-200 in combination with tislelizumab. PureTech expects to report topline Phase 1 results in the fourth quarter of 2021. In addition, PureTech plans to investigate LYT-200 as a single agent and in combination with other anti-cancer treatments, including chemotherapy and other immunotherapies.
Tislelizumab is potentially differentiated from the currently approved programmed cell death protein 1 (PD-1) antibodies in an engineered fragment crystallizable region (Fc region), which in preclinical studies has been shown to minimize potentially negative interactions with other immune cells. Tislelizumab has been approved in China for four solid tumor indications and regulatory decisions are pending for two additional indications. In January 2021, BeiGene and Novartis entered into a collaboration and license agreement granting Novartis rights to develop, manufacture and commercialize tislelizumab in North America, Europe and Japan.
“After a decade of optimizing use of immuno-oncology therapies, such as the checkpoint inhibitors that have certainly provided a paradigm shift in treating malignant diseases, we in the industry are eager to advance novel breakthrough agents and combinations to serve a wider range of cancer patients. Patients need new options as the first wave of immunotherapies work only in a small percentage of them. Well thought-out combination immunotherapy regimens may be that way forward,” said Aleksandra Filipovic, M.D., Ph.D., Head of Oncology at PureTech. “We believe LYT-200 has the potential to engage the immune system against what are currently intractable cancers, both as a single agent and in combination with checkpoint inhibitors. We look forward to evaluating whether LYT-200 in combination with BeiGene’s tislelizumab can improve outcomes for patients with metastatic solid tumors.”
Under the terms of the agreement, PureTech will maintain control of the LYT-200 program, including global R&D and commercial rights. BeiGene has agreed to supply tislelizumab for use in combination with LYT-200.
About LYT-200
LYT-200 is a monoclonal antibody targeting a foundational immunosuppressive protein, galectin-9, for the potential treatment of solid tumors, including pancreatic ductal adenocarcinoma, colorectal cancer and cholangiocarcinoma, that are difficult to treat and have poor survival rates. PureTech has presented preclinical data demonstrating high expression of galectin-9 across breast cancer, pancreatic cancer and cholangiocarcinoma samples and found that the highest levels of galectin-9 correlated with shorter time to disease relapse and poor survival. These data suggest that galectin-9 could be significant both as a therapeutic target for solid tumors for a range of cancers and as a cancer biomarker. Preclinical animal and patient-derived organoid tumor models also showed the potential efficacy of LYT-200 and the importance of galectin-9 as a target. LYT-200 is currently being evaluated in a Phase 1/2 adaptive design trial, and results from the Phase 1 portion are expected in the fourth quarter of 2021.
About Tislelizumab
Tislelizumab (BGB-A317) is a humanized IgG4 anti-PD-1 monoclonal antibody specifically designed to minimize binding to FcγR on macrophages. In pre-clinical studies, binding to FcγR on macrophages has been shown to compromise the anti-tumor activity of PD-1 antibodies through activation of antibody-dependent macrophage-mediated killing of T effector cells. Tislelizumab is the first drug from BeiGene’s immuno-oncology biologics program and is being developed internationally as a monotherapy and in combination with other therapies for the treatment of a broad array of both solid tumor and hematologic cancers.
The China National Medical Products Administration (NMPA) has granted tislelizumab in five indications, including full approval for first-line treatment of patients with advanced squamous non-small cell lung cancer (NSCLC) in combination with chemotherapy and for first-line treatment of patients with advanced non-squamous NSCLC in combination with chemotherapy; and conditional approval for the treatment of patients with classical Hodgkin’s lymphoma (cHL) who received at least two prior therapies, for the treatment of patients with locally advanced or metastatic urothelial carcinoma (UC) with PD-L1 high expression whose disease progressed during or following platinum-containing chemotherapy or within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy, and for the treatment of patients with hepatocellular carcinoma (HCC) who have received at least one systemic therapy. Full approval for these indications is contingent upon results from ongoing randomized, controlled confirmatory clinical trials.
In addition, two supplemental Biologics License Applications for tislelizumab have been accepted by the Center for Drug Evaluation (CDE) of the NMPA and are under review for second- or third-line treatment of patients with locally advanced or metastatic NSCLC who progressed on prior platinum-based chemotherapy and for patients with previously treated, locally advanced unresectable or metastatic microsatellite instability-high (MSI-H) or mismatch repair-deficient (dMMR) solid tumors.
BeiGene has initiated or completed 17 potentially registration-enabling clinical trials in China and globally, including 13 Phase 3 trials and four pivotal Phase 2 trials.
In January 2021, BeiGene and Novartis entered into a collaboration and license agreement granting Novartis rights to develop, manufacture, and commercialize tislelizumab in North America, Europe and Japan.
Tislelizumab is not approved for use outside of China.
About PureTech Health
PureTech is a clinical-stage biotherapeutics company dedicated to discovering, developing and commercializing highly differentiated medicines for devastating diseases, including inflammatory, fibrotic and immunological conditions, intractable cancers, lymphatic and gastrointestinal diseases and neurological and neuropsychological disorders, among others. The Company has created a broad and deep pipeline through the expertise of its experienced research and development team and its extensive network of scientists, clinicians and industry leaders. This pipeline, which is being advanced both internally and through PureTech's Founded Entities is comprised of 26 products and product candidates, including two that have received FDA clearance and European marketing authorization. All of the underlying programs and platforms that resulted in this pipeline of product candidates were initially identified or discovered and then advanced by the PureTech team through key validation points based on the Company's unique insights into the biology of the brain, immune and gut, or BIG, systems and the interface between those systems, referred to as the BIG Axis.
For more information, visit www.puretechhealth.com or connect with us on Twitter @puretechh.
Cautionary Note Regarding Forward-Looking Statements
This press release contains statements that are or may be forward-looking statements, including statements that relate to the company's future prospects, developments, and strategies. The forward-looking statements are based on current expectations and are subject to known and unknown risks and uncertainties that could cause actual results, performance and achievements to differ materially from current expectations, including, but not limited to, our expectations regarding the potential therapeutic benefits of our therapeutic candidates, our expectations regarding the potential therapeutic benefits of LYT-200 in patients with solid tumors, the expected timing of results from our Phase 1 trial of LYT-200 and the initiation of the Phase 2 trial, and the potential benefits of LYT-200 in combination with BeiGene’s anti-PD-1 therapy those risks, and uncertainties described in the risk factors included in the regulatory filings for PureTech Health plc. These forward-looking statements are based on assumptions regarding the present and future business strategies of the company and the environment in which it will operate in the future. Each forward-looking statement speaks only as at the date of this press release. Except as required by law and regulatory requirements, neither the company nor any other party intends to update or revise these forward-looking statements, whether as a result of new information, future events or otherwise.
View source version on businesswire.com: https://www.businesswire.com/news/home/20210707005110/en/
Contacts
Investors
Allison Mead Talbot
+1 617 651 3156
amt@puretechhealth.com
U.S. media
Stephanie Simon
+1 617 581 9333
stephanie@tenbridgecommunications.com













