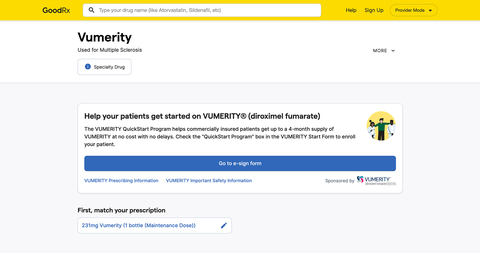
GoodRx’s just-announced Provider Mode makes it easier for providers to enroll patients on a specialty therapy via an electronic enrollment form
GoodRx (NASDAQ: GDRX), a leading consumer-focused digital healthcare platform, today announced a collaboration with Biogen (NASDAQ: BIIB) that aims to improve the patient and healthcare provider (HCP) experience when initiating a new specialty therapy. HCPs who have decided to start patients with relapsing forms of multiple sclerosis (MS) on VUMERITY® (diroximel fumarate) may now find the enrollment form and submit to the specialty hub through Provider Mode. Announced today, GoodRx’s Provider Mode is a new experience built specifically for HCPs to help them access solutions for the medications they prescribe.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20221013005410/en/
(Graphic: Business Wire)
MS affects more than one million people in the United States. Starting patients on specialty therapies to treat conditions such as MS can be an arduous process for HCPs in terms of paperwork and for the patients themselves, who can at times wait up to three weeks for a prescription to be filled. This new, digital enrollment in Provider Mode aims to expedite time to treatment initiation and significantly reduce the risk of documentation errors, which frequently occur when using traditional manual methods, including fax.
“One of the most important things we can do to improve the quality of healthcare today is to help providers find faster and easier ways to connect patients with the right drugs and treatments,” said Bansi Nagji, President of Healthcare at GoodRx. “We are proud to work with medical professionals to help patients with relapsing MS get the therapy they need more efficiently. This exciting integration with Biogen is just the start of the great work to come as we expand our offerings for providers.”
Built by GoodRx’s own team of medical professionals along with external healthcare provider focus groups, Provider Mode offers a redesigned workflow and a faster, more customized experience to help providers and office staff find the information they need in the moment. Since it started rolling out last December, it has seen an almost 90% opt-in rate from providers. Biogen is the first pharmaceutical manufacturer to utilize this feature in Provider Mode, allowing HCPs to send enrollment information directly to the specialty hub.
“We are proud to collaborate with GoodRx, a company that aligns with our commitment to help healthcare providers spend more of their time where it matters most – with their patients,” said Alisha A. Alaimo, President of Biogen's U.S. Organization.
To learn more about Provider Mode, go to www.goodrx.com/provider/join
About GoodRx
GoodRx is America’s digital resource for healthcare. Our technology delivers strong savings, trusted information and access to care to make healthcare affordable and convenient for all Americans. Since 2011, we have helped consumers save over $40 billion from pharmacy retail price and are one of the most downloaded medical apps over the past decade.
About Biogen
As pioneers in neuroscience, Biogen discovers, develops, and delivers worldwide innovative therapies for people living with serious neurological diseases as well as related therapeutic adjacencies. As one of the world’s first global biotechnology companies, Biogen was founded in 1978 by Charles Weissmann, Heinz Schaller, Sir Kenneth Murray, and Nobel Prize winners Walter Gilbert and Phillip Sharp. Today, Biogen has a leading portfolio of medicines to treat multiple sclerosis, has introduced the first approved treatment for spinal muscular atrophy, and developed the first and only approved treatment to address a defining pathology of Alzheimer’s disease. Biogen is also commercializing biosimilars and focusing on advancing one of the industry’s most diversified pipelines in neuroscience that will transform the standard of care for patients in several areas of high unmet need.
In 2020, Biogen launched a bold 20-year, $250 million initiative to address the deeply interrelated issues of climate, health, and equity. Healthy Climate, Healthy Lives™ aims to eliminate fossil fuels across the company’s operations, build collaborations with renowned institutions to advance the science to improve human health outcomes, and support underserved communities.
We routinely post information that may be important to investors on our website at www.biogen.com. Follow us on social media - Twitter, LinkedIn, Facebook, YouTube.
About VUMERITY® (diroximel fumarate)
VUMERITY is an oral fumarate with a distinct chemical structure from TECFIDERA® (dimethyl fumarate), approved in the U.S. for the treatment of relapsing forms of multiple sclerosis in adults, to include clinically isolated syndrome, relapsing-remitting disease and active secondary progressive disease. Once in the body, VUMERITY rapidly converts to monomethyl fumarate, the same active metabolite of dimethyl fumarate. VUMERITY is approved in more than 30 countries, and more than 20,000 patients have been treated with it, representing more than 15,000 patient-years of exposure across clinical trial use and patients prescribed VUMERITY.[i]
VUMERITY is contraindicated in patients with known hypersensitivity to diroximel fumarate, dimethyl fumarate or to any of the excipients of VUMERITY; and in patients taking dimethyl fumarate. Serious side effects for VUMERITY are based on data from dimethyl fumarate (which has the same active metabolite as VUMERITY) and include anaphylaxis and angioedema, progressive multifocal leukoencephalopathy, which is a rare opportunistic viral infection of the brain that has been associated with death or severe disability, a decrease in mean lymphocyte counts during the first year of treatment, herpes zoster and other serious infections, liver injury and flushing. The most common adverse events, obtained using data from dimethyl fumarate (which has the same active metabolite as VUMERITY), were flushing, abdominal pain, diarrhea and nausea.
Please click here for Important Safety Information and full Prescribing Information, including Patient Information for VUMERITY in the U.S.
GoodRx Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this press release that do not relate to matters of historical fact should be considered forward-looking statements, including without limitation statements regarding the benefits of the strategic agreement between GoodRx and Biogen or Provider Mode. These statements are neither promises nor guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, but not limited to, risks relating to our acquisition strategy, the integration of acquired business and the important factors discussed under the caption “Risk Factors” in GoodRx’s Annual Report on Form 10-K for the year ended December 31, 2021, and our other filings with the SEC. These factors could cause actual results to differ materially from those indicated by the forward-looking statements made in this press release. Any such forward-looking statements represent management’s estimates as of the date of this press release. While we may elect to update such forward-looking statements at some point in the future, we disclaim any obligation to do so, even if subsequent events cause our views to change.
Biogen Safe Harbor Statement
This news release contains forward-looking statements, including statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995, about the potential benefits of Provider Mode and the GoodRx platform; the potential benefits and results that may be achieved through our collaboration with GoodRx; the potential of our commercial business and pipeline programs; and our strategy and plans. These statements may be identified by words such as “aim,” “anticipate,” “believe,” “could,” “estimate,” “expect,” “forecast,” “goal,” “intend,” “may,” “plan,” “possible,” “potential,” “will,” “would” and other words and terms of similar meaning. You should not place undue reliance on these statements, or the scientific data presented.
These statements involve risks and uncertainties that could cause actual results to differ materially from those reflected in such statements, including without limitation, the risks of unexpected costs or delays; the risk of other unexpected hurdles; failure to protect and enforce our data, intellectual property and other proprietary rights and uncertainties relating to intellectual property claims and challenges; regulatory authorities may require additional information or further studies; third party collaboration risks; and the direct and indirect impacts of the ongoing COVID-19 pandemic on our business, results of operations and financial condition. The foregoing sets forth many, but not all, of the factors that could cause actual results to differ from our expectations in any forward-looking statement. Investors should consider this cautionary statement as well as the risk factors identified in our most recent annual or quarterly report and in other reports we have filed with the U.S. Securities and Exchange Commission. These statements are based on our current beliefs and expectations and speak only as of the date of this news release. We do not undertake any obligation to publicly update any forward-looking statements, whether as a result of new information, future developments or otherwise.
[i] Combined post-marketing data based on prescriptions and clinical trials exposure to VUMERITY as of December 31, 2021
View source version on businesswire.com: https://www.businesswire.com/news/home/20221013005410/en/
Contacts
GoodRx
Lauren Casparis
press@goodrx.com














