Abiomed (Nasdaq: ABMD) announces the U.S. Food and Drug Administration (FDA) has accepted and closed the post-approval study reports related to the pre-market approvals (PMA) for Impella heart pumps. The FDA’s action is another affirmation that Impella heart pumps are safe and effective for cardiogenic shock, high-risk PCI, post-cardiotomy cardiogenic shock, cardiogenic shock in the setting of myocarditis or cardiomyopathy, and right heart failure.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20221020005716/en/
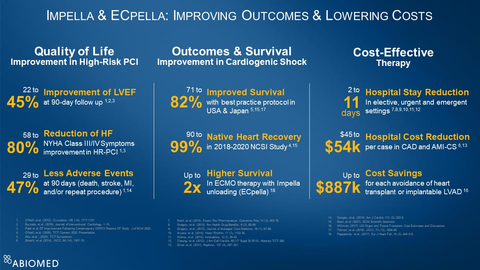
Figure 1
The FDA typically requires post-approval studies for medical devices that receive a PMA, the FDA’s highest level of regulatory approval. FDA post-approval studies use high-quality prospective data to confirm that the clinical study data submitted to the FDA to receive a PMA applies to a broader, real-world population of patients.
In total, Abiomed completed five post-approval studies for Impella over the seven years since its initial PMA was received. This large, multi-center experience was conducted at 46 sites and enrolled a total of 1,833 patients.
“This significant regulatory milestone once again confirms the safety and efficacy of Impella across a variety of clinical indications. I applaud the physician-researchers who led these studies and thank the patients who participated in them,” said Chuck Simonton, MD, Abiomed’s chief medical officer.
The totality of Impella data collected in the U.S., Europe and Japan demonstrates Impella improves outcomes and lowers costs. (see figure 1) This data includes:
- Impella-supported Protected PCI improves quality of life, with a 22% to 45% improvement in left ventricular ejection fraction at 90-day follow up1, 2, 3, a 58% to 80% reduction in New York Heart Association Class III and IV symptoms1, 3 and 29% to 47% fewer adverse events at 90 days1, 14.
- Impella improves outcomes in cardiogenic shock, with 71% to 82% survival with best practice protocols5, 15, 17, 90% to 99% native heart recovery in the 2018 to 2020 National Cardiogenic Shock Initiative (NCSI) Study4, 15 and up to two-times higher survival for ECMO therapy when it is combined with Impella unloading (known as ECpella)18.
- Impella is a cost-effective therapy that reduces hospital length-of-stay two to eleven days in elective, urgent and emergent settings7, 8, 9, 10, 11, 12, reduces hospital cost per case by $45,000 to $54,000 in coronary artery disease and AMI cardiogenic shock6, 13 and provides up to $887,000 in cost savings for each avoidance of a heart transplant or implantable LVAD16.
Impella is the most studied heart pump in the history of the FDA, with studies being conducted from 2006 to the present. Real-world data exists on nearly 200,000 Impella patients (see figures 2 & 3) and Impella is the subject of more than 1,200 peer-reviewed publications. It is included in 13 clinical society guidelines.
The clinical data and best practices learned from all Impella studies performed with the FDA, the Japanese Pharmaceuticals and Medical Devices Agency and in Europe, combined with prospective and real-world data, informed the design of the PROTECT IV and RECOVER IV randomized controlled trials. These on-label trials are designed to achieve the level of evidence for Impella to receive Class I guideline recommendations for high-risk PCI and AMI cardiogenic shock.
______________________________
- O’Neill, et al. (2012). Circulation, 126 (14), 1717-1727.
- Burzotta, et al. (2019). Journal of Interventional Cardiology, 1–10.
- Patel et al. EF Improvement Following Contemporary HRPCI: Restore EF Study. J of SCAI 2022.
- O’Neill, et al. (2020). TCT Connect 2020 Presentation.
- Ako, et al. (2022). TCT Symposium.
- Stretch, et al. (2014). JACC, 64 (14), 1407-15.
- Maini, et al. (2014). Expert Rev Pharmacoecon Outcomes Res, 14 (3), 403-16.
- Gregory, et al. (2013). Am Health Drug Benefits, 6 (2), 88-99.
- Gregory, et al. (2013). Journal of Managed Care Medicine, 16 (1), 61-69.
- Aryana, et al. (2014). Heart Rhythm, 11 (7), 1122-30.
- Wohns, et al. (2014). Innovations, 9 (1), 38-42.
- Cheung, et al. (2012). J Am Coll Cardiol, 60 (17 Suppl B) B110. Abstract TCT-385.
- Silver, et al. (2017). Nephron, 137 (4), 297–301.
- Dangas, et al. (2014). Am J Cardiol, 113 (2), 222-8.
- Basir, et al. (2021). SCAI Scientific Sessions.
- Milliman. (2017). US Organ and Tissue Transplant Cost Estimates and Discussion.
- Tehrani, et al. (2019). JACC, 73 (13), 1659-69.
- Pappalardo, et al. (2017). Eur J Heart Fail, 19 (3), 404-412.
ABOUT IMPELLA HEART PUMPS
Impella CP with SmartAssist® is U.S. FDA approved to treat certain advanced heart failure patients undergoing elective and urgent percutaneous coronary interventions (PCI), such as stenting or balloon angioplasty, to reopen blocked coronary arteries.
Impella CP with SmartAssist® and Impella 5.5® with SmartAssist® are U.S. FDA approved to treat heart attack or cardiomyopathy patients in cardiogenic shock, and have the unique ability to enable native heart recovery, allowing patients to return home with their own heart.
Impella RP® with SmartAssist is U.S. FDA approved to treat right heart failure or decompensation following left ventricular assist device implantation, myocardial infarction, heart transplant or open-heart surgery.
ABOUT ABIOMED
Based in Danvers, Massachusetts, USA, Abiomed (Nasdaq: ABMD) is a leading provider of medical technology that provides circulatory support and oxygenation. Our products are designed to enable the heart to rest and recover by improving blood flow and/or provide sufficient oxygenation to those in respiratory failure. For additional information, please visit abiomed.com.
FORWARD-LOOKING STATEMENTS
Any forward-looking statements are subject to risks and uncertainties such as those described in Abiomed's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
View source version on businesswire.com: https://www.businesswire.com/news/home/20221020005716/en/
Contacts
For further information:
Media:
Jenny Leary
Associate Director, U.S. Communications
+1 (978) 882-8491
jleary@abiomed.com
Investors:
Todd Trapp
Executive Vice President and Chief Financial Officer
+1 (978) 646-1680
ttrapp@abiomed.com













