Stryker (NYSE:SYK) today announced the launch of Citrefix™, a suture anchor system for foot and ankle surgical procedures. The new system uses Citregen™, an award-winning bioresorbable material designed to mimic the chemistry and structure of native bone.1
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20221213005214/en/
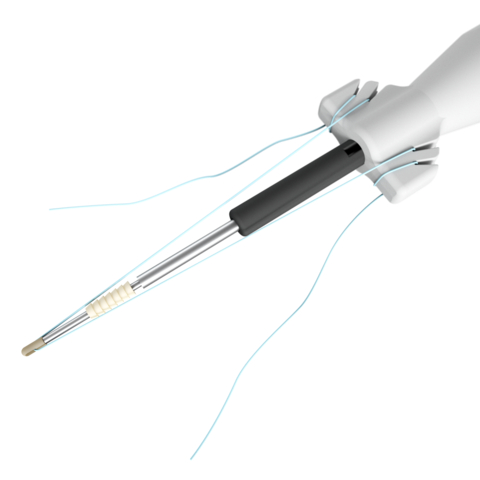
Stryker's new suture anchor system for foot and ankle surgical procedures, featuring the award-winning Citregen, a bioresorbable material designed to mimic the chemistry and structure of native bone. (Photo: Business Wire)
“Our customers will now benefit from the expanded use of one of the most innovative bioresorbable materials available for use in foot and ankle procedures,” said Michael Rankin, vice president, marketing and medical education for Stryker’s Foot & Ankle business. “Citrefix’s unique suture anchor system is the next step in our expanding Citregen portfolio.”
Citrefix is a disposable suture anchor system that features a resorbable biomimetic anchor body. It is made with Citregen, an elastomeric material made from a citrate polymer specially designed to mimic bone chemistry for controlled resorption without chronic inflammation.1 These unique chemical and mechanical properties are designed to help grafted tissue heal and healthy bone to grow when used in orthopaedic surgical applications.2 The sterile-packed set includes a cartridge with preloaded implant and eyelet, a drill bit, a drill guide and pre-assembled inserter.
“By leveraging Citregen’s unique material properties, Citrefix introduces design features that greatly increase its pull-out strength compared to other suture anchors,” said Wayne Berberian, M.D., orthopedic surgeon at the Ankle & Foot Institute in Maywood, N. J. “At the same time, the Citregen material benefits patients’ recovery since it is more easily accepted by the body, so they heal without suffering from the chronic inflammation that may occur with other bioresorbable suture anchors.”
After the successful launch of Citrelock last year, Citrefix is the second product in Stryker’s expanding portfolio using the material, with additional products expected in 2023. Citregen was awarded the 2022 Technology Innovation and Development Award by the Society for Biomaterials earlier this year.
For more information, visit footankle.stryker.com.
About Stryker
Stryker is one of the world’s leading medical technology companies and, together with its customers, is driven to make healthcare better. The company offers innovative products and services in Medical and Surgical, Neurotechnology, Orthopaedics and Spine that help improve patient and healthcare outcomes. Alongside its customers around the world, Stryker impacts more than 100 million patients annually.
More information is available at www.stryker.com.
A surgeon must always rely on his or her own professional clinical judgment when deciding whether to use a particular product when treating a particular patient. Stryker does not dispense medical advice and recommends that surgeons be trained in the use of any particular product before using it in surgery.
The information presented is intended to demonstrate the breadth of Stryker’s product offerings. A surgeon must always refer to the package insert, product label and/or instructions for use before using any of Stryker’s products. Products may not be available in all markets because product availability is subject to the regulatory and/or medical practices in individual markets. Please contact your sales representative if you have questions about the availability of products in your area.
___________________________________
- Society for Biomaterials, 2022 Technology Innovation and Development Award
- FDA 510(k) K200725
View source version on businesswire.com: https://www.businesswire.com/news/home/20221213005214/en/
Contacts
Andrea Sampson
President/CEO, Sampson Public Relations Group
asampson@sampsonprgroup.com
562.304.0301













