SYDNEY - Dec. 21, 2021 - PRLog -- OncoBeta® GmbH, a medical device company specialized in innovative epidermal radioisotope therapies announced today that the company has received ethics approval from the National Health and Medical Research Council (NHMRC) in Australia to begin the phase IV clinical study of Rhenium-SCT® to further evaluate the Complete Response Rate of patients with non-melanoma skin cancer.
The EPIC-Skin Study (Efficacy of Personalised Irradiation with Rhenium-SCT– for the treatment of non-melanoma skin cancer) will enrol 210 adult patients with a histological confirmation of stage I or II non-melanoma skin cancer to participate for 12 months, with a follow up period up to 24 months.
OncoBeta has contracted the Australian based Clinical Research Organization (CRO), Molecule2Market for this significant international, multicentre clinical study.
Dr. Gerhard Dahlhoff, Medical Director at OncoBeta stated, "This is a fantastic achievement. Receiving ethics approval marks an important milestone for this study, and the distribution of this therapy for non-melanoma skin cancer. It brings OncoBeta one step closer to helping safely and effectively treat patients around the world."
NHMRC ethics approval ensures the adherence to consistent safety monitoring and reporting of clinical trials that aligns with the Therapeutic Goods Administration (TGA) and guidance for Safety Monitoring and Reporting in Clinical Trials in relation to Australian and international research practices.
Shannon D. Brown III, CEO and Managing Director at OncoBeta, says, "We are dedicated to developing and improving world-class non-invasive therapies in the areas of dermatology and oncology for patients in need. This milestone ethics approval demonstrates our commitment to transparency, safety and improved patient quality of life."
Treatment with Rhenium-SCT has already been shown to be effective in the treatment of basal cell (BCC) and squamous cell carcinomas (SCC)1,2. Medical Director for OncoBeta Australia, Dr Saima Vohra, says, "This study will have a specific emphasis on Patient Reported Outcome Measures, and quality of life, as well as further confirmation of the efficacy of Rhenium-SCT for the treatment of NMSC."
The study will commence in January 2022, with the first patients scheduled for treatment with Rhenium-SCT at Royal North Shore Hospital in North Sydney. Clinic sites participating in the study include Austria, Germany, United Kingdom and five cities in Australia. Those interested in referring patients to the study, can contact OncoBeta directly at https://www.oncobeta.com/contact.
About the Rhenium-SCT® (Skin Cancer Therapy)
Non-melanoma skin cancer (NMSC) is the most common form of cancer in humans. The most common cause of NMSC is sun exposure, while other predisposing factors include genetic skin conditions and immunosuppressive diseases or treatments.3
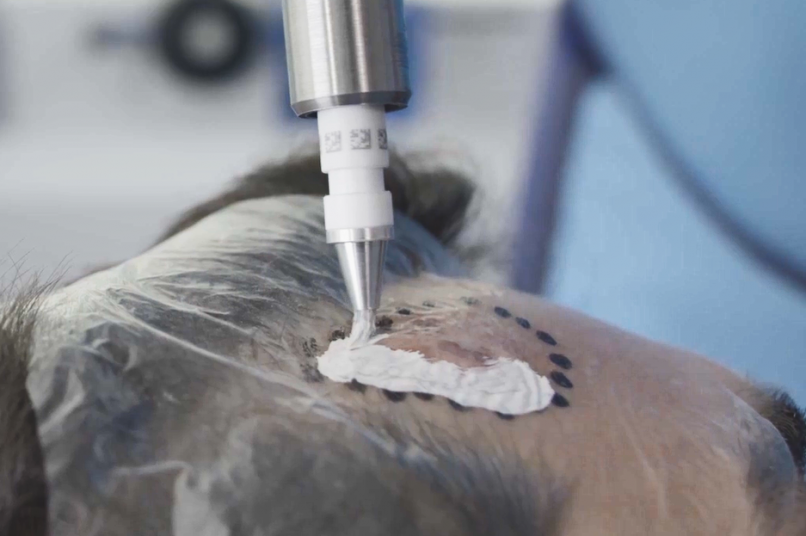
The Rhenium-SCT® is a painless*, single session†, non-invasive therapy providing for unparalleled aesthetic results, even in cases otherwise considered difficult to treat.2,4,5 The Rhenium-SCT utilizes the radioisotope Rhenium-188 in an epidermal application with optimal properties for the treatment of NMSCs (non-melanoma skin cancers). The Rhenium-SCT is a precise, personalised therapy that is only applied to the area needed to treat without affecting the healthy tissue. The specially designed device ensures the Rhenium-SCT compound never comes in direct contact with the patient's skin and the application is safe and simple for the applying physician. Most cases of NMSCs (Basal Cell Carcinomas and Squamous Cell Carcinomas) can be treated using the Rhenium-SCT in one single session†5. Scar-free healing5 of the treated lesion area and the regeneration of healthy tissue occurs usually within a few weeks after treatment5.
About OncoBeta®
OncoBeta®, with its headquarters located in Garching near Munich, Germany, is a privately held medical device company, specializing in the development and commercialization of state-of-the-art, innovative therapies. Since its foundation, OncoBeta has concentrated its efforts on the development, regulatory approval(s) and commercialization of the epidermal radioisotope therapy Rhenium-SCT® (Skin Cancer Therapy), targeting NMSCs. OncoBeta has perfected the customized application and device management system in conformity with all health, safety and environmental protection regulatory standards.
Find out more about the Rhenium-SCT® at www.oncobeta.com
Follow us on social media:
LinkedIn: www.linkedin.com/company/oncobeta-gmbh/
Facebook: www.facebook.com/oncobeta/
Instagram: www.instagram.com/oncobeta_gmbh/
Forward-looking statements
This announcement includes forward-looking statements that involve risks, uncertainties and other factors, many of which are outside of OncoBeta's control, and which could cause actual results to differ materially from the results discussed in the forward-looking statements. Forward-looking statements include statements concerning OncoBeta's plans, objectives, goals, future events, performance and/or other information that is not historical information. All such forward-looking statements are expressly qualified by these cautionary statements and any other cautionary statements which may accompany the forward-looking statements. OncoBeta undertakes no obligation to publicly update or revise forward-looking statements to reflect subsequent events or circumstances after the date made, except as required by law.
*No reported pain2,4
†Complete tumour regression in 98.5% of lesions treated, with 89% after a single application5
References
- Castellucci P, et al. Eur J Nucl Med Mol Imaging. 2021; 48: 1511–1521.
- Cipriani C, et al. J Dermatolog Treat. 2020; Jul 22:1-7.
- Cancer.net. Skin Cancer (Non-Melanoma): Risk Factors and Prevention. October 2020. https://www.cancer.net/cancer-types/skin-cancer-non-melanoma/risk-factors-and-prevention (accessed October 2021).
- Sedda AF, et al. Clin Exp Dermatol. 2008;33(6):745-749.
- Cipriani C, Sedda AF. Epidermal Radionuclide Therapy - Dermatological High-Dose-Rate. Brachytherapy for the Treatment of Basal and Squamous Cell Carcinoma. In: Therapeutic Nuclear Medicine, editor Baum RP; New York: Springer, 2014
Contact
Jane Morey (Morey Media / Showoff Group)
***@moreymedia.com.au
Photos: (Click photo to enlarge)


Read Full Story - OncoBeta receives ethics approval for EPIC-Skin study to evaluate Rhenium-SCT in Australia | More news from this source
Press release distribution by PRLog














