FAYETTEVILLE, Ark. - Aug. 25, 2022 - PRLog -- Lineus Medical announces publication of the randomized controlled trial performed at Colorado State University Veterinary Teaching Hospital in the Journal of the American Veterinary Medical Association. The canine study had a total of 367 patients and documented the impact that a new class of peripheral IV breakaway devices had on IV complications in hospitalized dogs. The results of the study found that the use of SafeBreak Vascular Vet, Lineus Medical's break-away device, reduced IV complications by 65%.1 Additionally, the results indicated that SafeBreak use may also limit patient discomfort, owner (client) expense, and staff time devoted to managing IV complications.1
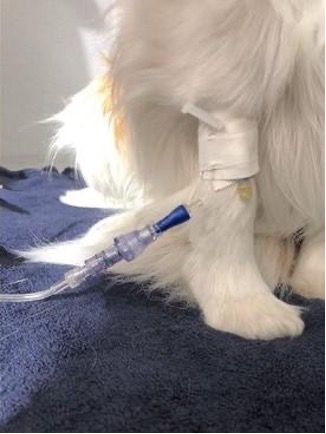
SafeBreak® Vascular Vet is a breakaway device that removes harmful forces from peripheral IV lines. The device is installed between the long IV tubing from the pump, and the patient's IV catheter. When harmful forces are applied to the IV line, SafeBreak separates to protect the patient and the IV line. SafeBreak has been proven to reduce IV complications in both small and large animals2. Lineus Medical sponsored the study to document the impact that SafeBreak has on IV complications rates compared to the standard of care. The study demonstrated that there was a statistically significant reduction in overall IV complications when SafeBreak was used. This included reducing IV-line breakage by 92%, dislodgement by 80%, phlebitis by 86%, and occlusion by 100%.1
"Reducing peripheral IV complications is imperative as it ensures that the patient receives all prescribed treatments in a timely manner, but it also may reduce the risk for venous depletion, reduce client costs, and minimize pain for the dog," said Sydney Simpson, co-author of the study.
Dr. Kristin Zersen, principal investigator and senior study author, adds, "This study highlighted a tremendous clinical benefit where the use of SafeBreak Vascular Vet decreased the overall IV complication rate by 65%. With the cost of replacing an IV around $60 compared to the $6.90 cost to replace SafeBreak, this reduction in complications equates to a significant cost savings to the client.1 Just as important, it reduces the need for IV replacement, which can cause pain and anxiety for our patients."
"We were thrilled that the results from this canine study were so significant. In veterinary medicine, the way procedures and supplies are billed to the pet owner is very straight forward compared to a human hospital. This allowed us to clearly show that reducing IV complications with SafeBreak delivers a direct clinical and financial benefit for patients, clients, and care providers," said Spencer Jones, Founder and Chief Technology Officer at Lineus Medical.
ABOUT LINEUS MEDICAL
Lineus Medical is the developer of a unique, patented, break-away technology designed to help reduce complications associated with a variety of medical tubing, such as feeding tubes, urinary catheters and other medical lines. More information about Lineus Medical can be found at www.lineusmed.com. Follow Lineus Medical on LinkedIn, Twitter, or Instagram.
MKG 0158 Rev 00
References
1. Simpson, S. E., & Zersen, K. M. (2022). Fewer peripheral intravenous catheter complications in hospitalized dogs when force-activated separation devices are used versus not used in a randomized controlled clinical trial. Journal of the American Veterinary Medical Association, 1–6. https://doi.org/10.2460/javma.22.03.0125
2. University of Tennessee Large Animal Study Data on File
Contact
Vance Clement
***@lineusmed.com
Photos: (Click photo to enlarge)![]()

Read Full Story - Lineus Medical Announces Randomized Controlled Trial Publication in Journal of the American Veterinary Medical Association | More news from this source
Press release distribution by PRLog
Lineus Medical Announces Randomized Controlled Trial Publication in Journal of the American Veterinary Medical Association
August 25, 2022 at 13:01 PM EDT













